The focus of Dr. Regan’s Investigational Pathology laboratory is to increase our understanding of the interplay between the immune system and (non-immune) tumor stroma, and how these compartments of the tumor microenvironment promote metastasis as well as respond to and mediate extrinsic mechanisms of resistance to anti-cancer therapy. To investigate this area of cancer biology, his laboratory utilizes a combination of in vitro 3-dimensional tumor co-culture models and animal models, focusing on breast and bone cancer (osteosarcoma). Dr. Regan also has a strong interest in comparative oncology and leveraging naturally occurring cancers in dogs as both a surrogate and intermediary model to evaluate and validate his laboratory’s investigations into the tumor microenvironment.
Services
- Project design and consultation
- Interpretation of clinical pathological tests (complete blood count, serum chemistry analysis, urinalysis, etc.)
- Necropsy, dissection and trimming
- Sample preparation and submission including decalcification
- Histopathological and cytological evaluation (includes grading, scoring, etc.)
- Immunohistochemistry (IHC), Immunocytochemistry (ICC), and Immunofluorescence (IF)
- IHC, ICC and IF protocol development
- Western Blot/Dot Blot with quantification
- Droplet Digital PCR protocol development
- Image collection, analysis and digital pathology
- Data collection and interpretation
Projects
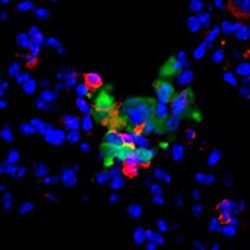
Evaluation of losartan as a re-purposed monocyte migration inhibitor and anti-metastatic agent in canine and human osteosarcoma.
In collaboration with the Dow laboratory in the Flint Animal Cancer Center, we study the effects of the angiotensin receptor blocker losartan on CCR2 signaling, tumor-mediated monocyte recruitment and macrophage phenotype utilizing mouse metastasis models and dogs with naturally occurring osteosarcoma. These studies are ongoing, but initially published results from this work has led to a Phase I clinical trial of losartan in human osteosarcoma patients, in collaboration with Carrye Cost and Kelly Faulk at Children’s Hospital Colorado.
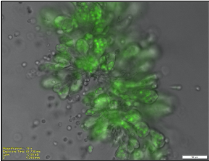
Understanding the role of lung fibroblasts in modulating extrinsic chemoresistance in human breast cancer.
Utilizing primary donor lung fibroblasts and a 3-D co-culture model, we are assessing how one of the most abundant stromal cells from a common site of breast cancer metastasis influences chemotherapy drug response for both conventional and experimental anti-cancer therapeutics. This project utilizes live-cell imaging and molecular approaches (flow cytometry, kinase arrays, multiplex cytokine analysis) to understand the cross-talk between tumor cells and fibroblasts, with the goal of identifying rational drug-combinations that reverse this chemoresistance and can be moved forward for screening in animal cancer models.
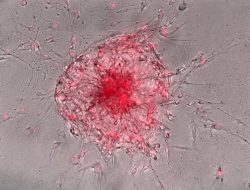
Characterizing the role of tumor-derived extracellular vesicles in regulating alveolar macrophages and osteosarcoma lung metastasis.
Our laboratory is studying the bio-distribution of human osteosarcoma extracellular vesicles in the lung and their role in regulating alveolar macrophages as a potential diagnostic and therapeutic target for osteosarcoma metastasis. These studies leverage a combination of approaches utilizing human primary cells, mouse models, and dogs with naturally occurring osteosarcoma.
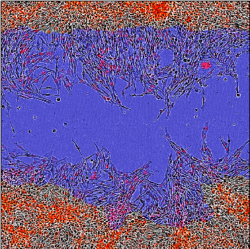
Targeting integrin signaling in canine and human osteosarcoma.
We are performing comparative in vitro investigations in human and canine osteosarcoma cell culture models evaluating the anti-neoplastic effects of small molecule compounds targeting different levels of the integrin signaling cascade. These drug screening studies are being conducted both alone, and in combination with standard of care drugs currently utilized for the treatment of osteosarcoma, in hopes of identifying a new and effective combination therapy that can be assessed in dogs with spontaneous osteosarcoma. These studies utilize a combination of live-cell imaging and biochemical assays to investigate these effects.
Defining the immunological landscape of translationally-relevant canine cancers.
Recent developments in human oncology have dramatically increased the relevance of cancer immunotherapy as a new treatment modality. However, further advances in the field of immuno-oncology will require continued identification of predictors of patient response to, and pre-clinical investigations of, rational therapeutic combinations of these drugs. Current mouse tumor models have intrinsic deficiencies in predicting microenvironmental and molecular determinants of response to immunotherapy. In contrast, naturally occurring tumors in dogs have significant potential to inform human immunotherapy studies due to their spontaneous development under the selective pressure of an intact immune system, allowing for a natural evolution of the processes of immune evasion, therapy resistance, and metastasis. Yet at present, our understanding of the basic immune landscape of common canine tumors is significantly lacking. Thus, in collaboration with the Dow and Duval laboratories at the Flint Animal Cancer Center, our goal is to increase our understanding of the basic immune landscape of common and translationally canine tumors.
About Dr. Regan

Dr. Regan received his DVM degree from the University of Georgia in 2011. Following, he completed his residency training in veterinary anatomic pathology and a Ph.D. in comparative pathology in the Department of Microbiology, Immunology, and Pathology at Colorado State University. In 2018 he accepted his current faculty position in the Flint Animal Cancer Center and the Department of Microbiology, Immunology, and Pathology at Colorado State University.
Contact

Investigational Pathology Lab
Assistant Professor, Department of Microbiology, Immunology, and Pathology